GRK1896 member contributes via in situ TEM to microscopic understanding of SCALMS catalysts
A collaborative “Highly Effective Propane Dehydrogenation Using Ga−Rh Supported Catalytically Active Liquid Metal Solutions” has been recently published in ACS Catalysis. This work was contributed by a team of scientist in Erlangen including Chemists, Experimental/Theoretical Physcists, Materials Scientist from many departments and institute lead by Prof. Peter Wasserscheid from the Institute of Chemical Reaction Engineering and Helmholtz-Institute Erlangen-Nuremberg for Renewable Energy. In this work, TEM, particularly in situ observations, has contributed an important part to get insight and derive a microscopic understanding to the composition and phase stability of the Ga-Rh nano particles. As an important step to explore Supported Catalytic Active Liquid Metal Solutions (SCALMS), this work demonstrated that rhodium (Rh), an element that has barely been reported as an active metal for selective dehydrogenation of alkanes becomes a very active, selective, and robust dehydrogenation catalyst when exposed to propane in the form of single atoms at the interface of a solid-supported, highly dynamic liquid Ga−Rh mixture.
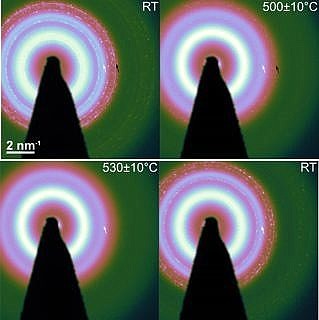
SCALMS have been recently demonstrated to be a highly promising class of heterogeneous catalysts, which show superior activity, robust performance and selectivity in dehydrogenation reactions. At typical dehydrogenation temperatures of 500 °C, it is conceived that the large surface to volume ratio of the nano-droplets of the liquid metals, supported in the open meso- or macroporous scaffold, provide huge number of catalytically active sites which are intrinsically robust to coke formation and degradation due to the highly dynamic nature of the liquid metal alloy. Such liquid metal nanoalloys are intermetallic compounds of low-melting host elements (e.g., Ga, In) with small amount of catalytically active transition metal elements. These intermetallic compounds typically have complex stable crystal phases in solid state. The structural information, particularly the phase stability of the nanoalloys, is indispensable to render the full picture of their catalytic performance.
- , , , , , , , , , , , , , , , :
Highly Effective Propane Dehydrogenation Using Ga-Rh Supported Catalytically Active Liquid Metal Solutions
In: ACS Catalysis (2019), p. 9499-9507
ISSN: 2155-5435
DOI: 10.1021/acscatal.9b02459
BibTeX: Download