Simon Kraschewski
Dr.-Ing. Simon Kraschewski
Project description
The process of metal-induced crystallization describes the low temperature crystallization of amorphous semiconductors, for example Si (a-Si), in the presence of certain metals, like Al, which form a eutectic system with Si [1]. The phenomenon is exploited in the so-called Al-induced layer exchange (AlILE) process enabling fabrication of thin polycrystalline Si films with large grain size on cheap substrates. In AlILE a layered stack of Al and a-Si with a thin AlOx interlayer is annealed at 180-550 °C resulting in a complete layer exchange of the Si and Al layer accompanied by crystallization of Si (c-Si) in the initial place of the Al layer. According to the literature [1-4] AlILE is based on the interplay of 5 elementary processes which are illustrated in Figure 1.
The AlILE process can be enhanced using a Ti/TiOx layer as barrier layer, this leads to up to 250 µm [5]. Figure 2 shows an in situ series of STEM images. The occurence of a secondary phase (Ti(Al Si)3) can be observed before the layer exchange starts. This secondary phase plays a majore role in the suppression of nucleation of new Si nuclei, so the Si nuclei have much more space to grow compared with normal AlILE.
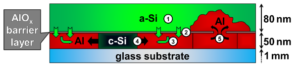
Fig.1: Mechanism of the AlILE process: 1. Dissolution of Si atoms in the amorphous Si (a-Si) layer, 2. Diffusion of Si atoms through AlOx barrier layer, 3. Diffusion of Si atoms through Al, 4. Crystallization of Si (c-Si) followed by lateral growth and 5. Transport of Al (“Push up”) of Al into top layer.

Fig. 2: In situ experiments on Ti.AlILE: At first the formation of the Ti enriched secondary phase and after that the Si crystallization due to the layer exchange.
References
[1] O. Nast et al., Appl. Phys. Lett. 73, (1998).
[2] P. I. Widenborg and A. G. Aberle, J. Cryst. Growth 242 (2002).
[3] A. Sarikov et al., J. Non-Cryst. Solids 352 (2006).
[4] B. I. Birajdar et al., Scr. Mater. 66 (2012).
[5] T. Antesberger, et al., J. Appl. Phys. 112 (2012)

